Introduction:
In order to modify the immune response balance between donor and recipient in the context of haploidentical transplantation, the use of post-transplant cyclophosphamide (PTCy), which reduces the population of recipient alloreactive T cells, has become the preferred strategy for graft-versus-host disease (GVHD) prophylaxis. Recently, this approach has also been tested and validated in HLA-matched related or unrelated transplants, as well as in unrelated transplants with HLA mismatch. However, high doses of PTCy can cause cardiac toxicity, hemorrhagic cystitis, and potentially increase the incidence of viral infections. Therefore, comparative studies are needed to evaluate the outcomes of PTCy use compared to other conventional GVHD prophylaxis strategies.
Objective:
To evaluate the outcomes of patients undergoing allogeneic peripheral blood stem cell transplantation (PBSCT) and to compare the use of post-transplant cyclophosphamide with other GVHD prophylaxis strategies.
Patients and Methods:
Retrospective analysis of consecutive patients transplanted between March 2014 and March 2023 at two transplant centers. The median follow-up was 24 months (range: 3-116 months).
Results:
A total of 118 adult patients undergoing PBSCT were included. There were 70 HLA-matched related donors, 28 HLA-matched unrelated donors, and 20 unrelated donors with one HLA mismatch. The median age was 52 years (range: 18 to 80), with a majority being male (51%). The most common diagnoses were acute myeloid leukemia (37%), acute lymphoblastic leukemia (18%), and myelodysplastic syndrome (18%). The disease risk index (DRI) was considered low or intermediate in 53% of cases, high in 31%, and very high in 16%. The majority of patients (76%) had a low risk of transplant-related complications (HCT-CI). Most patients received reduced-intensity conditioning regimens (78%), and the majority (72%) received some sort of T-cell depletion, with 36 (31%) patients receiving PTCy and 49 (41%) receiving ATG.
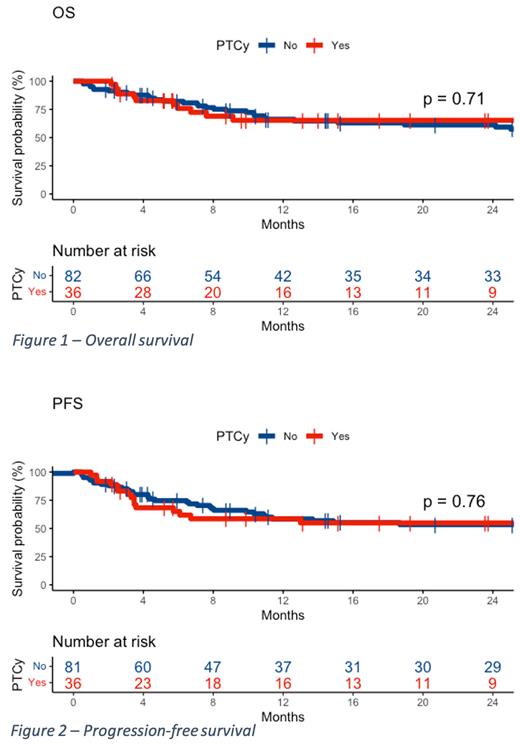
No significant differences were found between the groups (PTCy vs. non-PTCy) in overall survival at 24 months (65% vs. 61%, p = 0.71, respectively), disease-free survival at 24 months (55% vs. 53%, p = 0.76), non-relapse mortality at 24 months (21% vs. 23%, p = 0.84), relapse rate at 24 months (24% vs. 24%, p = 0.61), neutrophil engraftment at 30 days (89% vs. 93%, p = 0.49), and incidence of grade II-IV acute GVHD at 100 days (31% vs. 29%, p = 0.99), grade III-IV acute GVHD at 100 days (6% vs. 12%, p = 0.22), chronic GVHD at 24 months (42% vs. 50%, p = 0.51), and moderate/severe chronic GVHD at 24 months (19% vs. 23%, p = 0.66). There was also no difference in the incidence of infections, including viral infections and cytomegalovirus reactivation.
Conclusions:
The use of PTCy demonstrated similar clinical outcomes to other GVHD prophylaxis strategies, without an increased risk of infections or mortality. Therefore, PTCy can be considered a viable, safe, and cost-effective option for GVHD prophylaxis in the context of HLA-matched and HLA-mismatched transplants.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal